Your CPAP Ventilator Recall Attorney is Ready to Fight For You
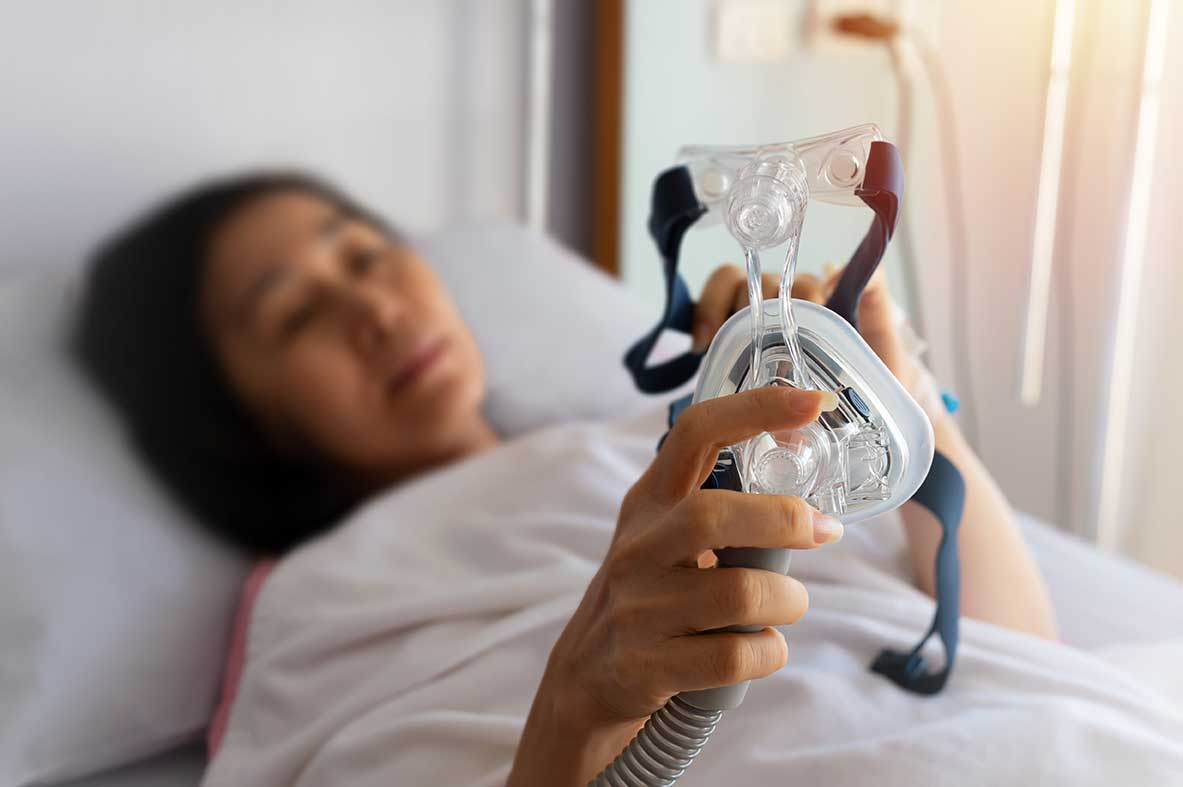
Philips Respironics® recalled certain DreamStation® Ventilators, CPAP, and BiLevel® PAP devices in June 2021 for potential health risks--the attorneys at DLG For Me are investigating claims that these recalled CPAP and ventilator devices are causing serious injuries, including cancer.
The Reason for the CPAP Ventilator Recall
Philips® recalled their DreamStation® devices when it discovered the sound abatement foam inside the machines can degrade into black debris over time and be inhaled or ingested by users. The polyester-based polyurethane (PE-PUR) sound abatement foam is used to reduce sound and vibration while the device is in use. The foam can also off-gas dangerous chemicals that are inhaled by patients.
Philips® has said that foam degradation increases with the use of unapproved cleaning systems and in high humidity environments.
CPAP, BiPap, and Ventilator Uses
CPAPs and BPAPs are used to manage sleep apnea by forcing air through the patient’s airways.
Sleep apnea is a serious sleep disorder that causes the breath to repeatedly stop and start throughout the night.
People with sleep apnea snore loudly and often do not feel rested the next day.
The three main kinds of sleep apnea are obstructive sleep apnea, central sleep apnea, and complex sleep apnea syndrome.
The most common is obstructive sleep apnea, where the throat muscles relax and cause the patient to stop breathing.
Symptoms of sleep apnea are not especially alarming in isolation but over time can lead to serious negative health consequences including heart problems.
If you have any of these symptoms, you might have sleep apnea.
- Loud snoring
- Gasping for air during sleep
- Waking with a dry mouth
- Morning headaches
- Insomnia
- Daytime fatigue
- Irritability
CPAP and BiLevel PAPs treat sleep apnea by forcing air through the airways so the patient does not stop and start breathing continuously throughout the night. Unfortunately, some of the devices on the market can cause other serious health problems.
The Dangerous Effects of Using Recalled CPAPs, BPAPs, or Ventilators
Using the recalled devices might be linked to serious life-threatening conditions, including but not limited to:
- Leukemia
- Multiple myeloma
- Lung disease
- Asthma
- Sarcoidosis
- Kidney disease
- Liver disease
- Pneumonia
- Breast cancer
- Sinus cancer
- Throat cancer
- Prostate cancer
Use of the recalled Philips® CPAP, BiPAP, and ventilator can also cause side effects like breathing problems, inflammation, headaches, dizziness, nausea, vomiting, and asthma. If you have experienced any of these side effects after using a recalled device, it could be a sign that you have sustained a more serious injury.
What to do if You Have Used a Recalled CPAP, BPAP, or Ventilator
The FDA recommends that users of recalled CPAP and BiPAP users talk to their doctors to determine the right course of action. Users of recalled devices should register their devices on the Philips Respironics® recall website.
For ventilator users, the FDA recommends that you not discontinue use of the ventilator until getting advice from your doctor. You should also register your recalled device on the Philips Respironics® recall website.
Using one of the recalled devices for more than 6 months increases your chance of injury. Many injured consumers are now filing lawsuits claiming compensation for damages from using Philips DreamStation® CPAPs, BiLevel® PAPs, or mechanical ventilators.
If you suspect that you were injured by using one of these recalled devices, contact a DLG For Me CPAP ventilator recall attorney today for a complimentary consultation because your time to file a claim is limited. The team at DLG For Me has decades of experience holding large corporations accountable for their dangerous products and can help you get the compensation you deserve.
FAQs
What is a CPAP?
A continuous positive airway pressure machine, also known as a CPAP, is used to treat sleep apnea by providing a continuous stream of air through the attached mask.
Which CPAPs were recalled?
Philips Respironics® recalled these DreamStation® CPAP and BiLevel® PAP devices:
- DreamStation ASV®
- DreamStation ST®, AVAPS®
- SystemOne ASV4®
- C-Series ASV®
- C-Series S/T® and AVAPS®
- OmniLab Advanced+®
- SystemOne® (Q-Series®)
- DreamStation®
- DreamStation Go®
- Dorma 400®
- Dorma 500®
- REMstar SE Auto®
If my CPAP was recalled, what should I do?
If your CPAP was recalled, the manufacturer recommends discontinuing use and consulting your physician or durable medical equipment provider about an alternative therapy.
What is a BiLevel® PAP?
A BiLevel® PAP, BiPAP, or BPAP is a positive airway pressure machine that pumps higher air pressure into the airways when you breathe in and lower pressure when you breathe out.
Which BiLevel® PAPs were recalled?
Philips Respironics® recalled the following CPAPs and BiPaps:
- DreamStation ASV®
- DreamStation ST®, AVAPS®
- SystemOne ASV4®
- C-Series ASV®
- C-Series S/T® and AVAPS®
- OmniLab Advanced+®
- SystemOne® (Q-Series®)
- DreamStation®
- DreamStation Go®
- Dorma 400®
- Dorma 500®
- REMstar SE Auto®
If my BiLevel® Pap was recalled, what should I do?
As with recalled CPAPs, the manufacturer recommends discontinuing use of the recalled device and consulting with your doctor about possible alternative therapies.
What is a ventilator?
A ventilator is a device that assists the patient’s breathing by delivering breathing gas containing oxygen.
Which Philips® ventilators were recalled?
Philips Respironics® recalled the following ventilators:
- Trilogy 100®
- Trilogy 200®
- Garbin Plus®, Aeris®, LifeVent®
- A-Series BiPAP A40®
- A-Series BiPAP A30®
If my ventilator was recalled, what should I do?
For patients using life-sustaining recalled ventilators, the manufacturer does not recommend discontinuing use until you have consulted with your doctor. At the discretion of your physician, the benefits of continued usage of the recalled device may outweigh the risks identified on the recall notification.
The following article will give you more information on how a DLG For Me CPAP ventilator recall attorney can assist you in your claim.
$500 Million+
Won in 2020 Alone
CPAP Recall Case Review
Top-Rated & Reviewed
DLG For Me Awards and Recognition